
He chlorination of methane occurs in a number of steps that results in the formation of chloromethane and hydrogen chloride. the overall reaction is 2ch4(g)+3cl2(g)⟶2ch3cl(g)+2hcl(g)+2 cl−(g) 2ch4(g)+3cl2(g)⟶2ch3cl(g)+2hcl(g)+2 cl−(g) suppose that a chemist combines 295 ml295 ml of methane and 725 ml725 ml of chlorine at stp in a 2.00 l2.00 l flask. the flask is then allowed to stand at 298 k.298 k. if the reaction reaches 77.0%77.0% completion, what is the total pressure in the flask?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:20, mathman783
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 13:00, jaylanmahone223
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Do you know the correct answer?
He chlorination of methane occurs in a number of steps that results in the formation of chloromethan...
Questions in other subjects:


Mathematics, 30.11.2020 03:00

Mathematics, 30.11.2020 03:00


Mathematics, 30.11.2020 03:00






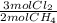 = 0,0198 moles Cl₂. That means that 0,0324-0,0198 = -0,0126 moles of Cl₂ are in excess-.
= 0,0198 moles Cl₂. That means that 0,0324-0,0198 = -0,0126 moles of Cl₂ are in excess-.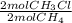 = 0,0102 moles of CH₃Cl -that are the same moles of HCl and Cl⁻-
= 0,0102 moles of CH₃Cl -that are the same moles of HCl and Cl⁻-



