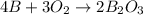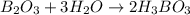Chemistry, 23.10.2019 18:30, jasmin2344
When elemental boron, b, is burned in oxygen gas, the product is diboron trioxide. if the diboron trioxide is then reacted with a measured quantity of water, it reacts with the water to form what is commonly known as boric acid, b(oh)3. write a balanced chemical equation for each of these processes. (use the lowest possible coefficients. omit states-of-matter in your answer.)

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 10:30, Riplilpeep
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1
Do you know the correct answer?
When elemental boron, b, is burned in oxygen gas, the product is diboron trioxide. if the diboron tr...
Questions in other subjects:

Mathematics, 11.09.2021 06:50

Mathematics, 11.09.2021 06:50

Social Studies, 11.09.2021 06:50




Mathematics, 11.09.2021 06:50

Social Studies, 11.09.2021 06:50