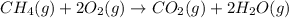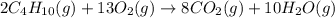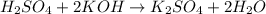Chemistry, 23.10.2019 17:50, dxnimxriee
Methane ch4 (g) reacts with oxygen gas to produce carbon dioxide and water. b) butane c4h10 (g) reacts with oxygen gas to produce carbon dioxide and water. c) an aqueous solution of sulfuric acid reacts with aqueous potassium hydroxide to produce potassium sulfate and water.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 12:00, jackfooman3100
Explaining why atoms bondcomplete the sentence. atoms form chemical bonds to satisfy the rule and to become .
Answers: 1

Chemistry, 23.06.2019 13:30, querline87
How many more valence electrons does sodium need to have a full outer valence shell
Answers: 1
Do you know the correct answer?
Methane ch4 (g) reacts with oxygen gas to produce carbon dioxide and water. b) butane c4h10 (g) rea...
Questions in other subjects:


Biology, 22.08.2019 01:00



Mathematics, 22.08.2019 01:00


Biology, 22.08.2019 01:00



Mathematics, 22.08.2019 01:00