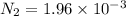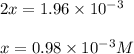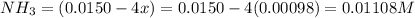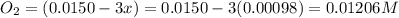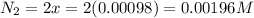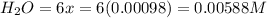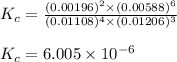Chemistry, 23.10.2019 17:00, tasnimabdallah971
Be sure to answer all parts. the first step in hno3 production is the catalyzed oxidation of nh3. without a catalyst, a different reaction predominates: 4 nh3(g) +3 o2 (g) ⇌ 2 n2(g) + 6 h2o(g) when 0.0150 mol of nh3(g) and 0.0150 mol of o2(g) are placed in a 1.00−l container at a certain temperature, the n2 concentration at equilibrium is 1.96 × 10−3 m. calculate kc.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, Unknowndragon42
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 19:30, 2020sanchezyiczela
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
Do you know the correct answer?
Be sure to answer all parts. the first step in hno3 production is the catalyzed oxidation of nh3. wi...
Questions in other subjects:

Mathematics, 24.06.2019 23:00




History, 24.06.2019 23:00

Mathematics, 24.06.2019 23:00

Mathematics, 24.06.2019 23:00

English, 24.06.2019 23:00


Mathematics, 24.06.2019 23:00


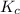 for the reaction is
for the reaction is 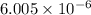
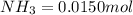
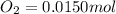
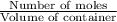
![[NH_3]_i=\frac{0.0150}{1.00}=0.0150M](/tpl/images/0342/7531/438ea.png)
![[O_2]_i=\frac{0.0150}{1.00}=0.0150M](/tpl/images/0342/7531/0aac8.png)
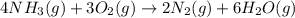
![K_c=\frac{[N_2]^2[H_2O]^6}{[NH_3]^4[O_2]^3}](/tpl/images/0342/7531/b62d0.png) .......(1)
.......(1)