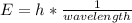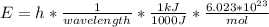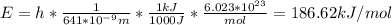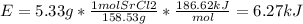Chemistry, 23.10.2019 17:00, camirialchambers17
In fireworks, the heat of the reaction of an oxidizing agent, such as kclo4, with an organic compound excites certain salts, which emit specific colors. strontium salts have an intense emission at 641 nm. what is the energy (in kj) of this emission for 5.33 g of the chloride salt of strontium? assume that all the heat produced is converted to emitted light. enter to 2 decimal places.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, sleimanabir
Balance this equation: n2 + h2 > nh3, write the following molar ratios: n2 / n2 / nh3 h2 /
Answers: 1

Chemistry, 22.06.2019 19:00, andrecoral105
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 22.06.2019 21:20, paatnguyyen
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(ii) carbonate, in concentrated sulfuric acid. the sulfuric acid reacts with the copper(ii) carbonate to produce a blue solution of copper(ii) sulfate. scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: (s) (aq) (s) (aq) suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. he adds powdered iron to a copper(ii) sulfate sample from the plant until no more copper will precipitate. he then washes, dries, and weighs the precipitate, and finds that it has a mass of .
Answers: 2

Chemistry, 22.06.2019 21:20, jordan2875
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1
Do you know the correct answer?
In fireworks, the heat of the reaction of an oxidizing agent, such as kclo4, with an organic compoun...
Questions in other subjects:




English, 04.04.2021 16:30

Biology, 04.04.2021 16:30

Mathematics, 04.04.2021 16:30

Mathematics, 04.04.2021 16:30


Mathematics, 04.04.2021 16:30