
Chemistry, 16.09.2019 05:50, Bleejones00
Write a balanced chemical equation for the reaction of zncl2 with excess naoh to produce na2zn(oh)4 sodium zincate. what mass of sodium zincate can be produced from 2.00 g of zncl2 with excess naoh by this reaction?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, WinterStrikesBack
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 12:30, kingbot350
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 13:00, taylorpayne525p8qxky
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2
Do you know the correct answer?
Write a balanced chemical equation for the reaction of zncl2 with excess naoh to produce na2zn(oh)4...
Questions in other subjects:


History, 23.07.2019 04:00


Social Studies, 23.07.2019 04:00




History, 23.07.2019 04:00



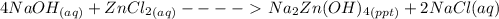
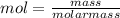
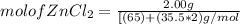
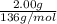
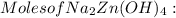
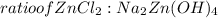
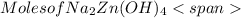 = 0.0147 mol
= 0.0147 mol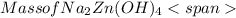 = [(23 * 2)+(65)+(16 * 4) + (1*4)] g/mol * 0.0147 mol
= [(23 * 2)+(65)+(16 * 4) + (1*4)] g/mol * 0.0147 mol



