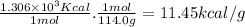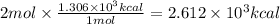Combustion reactions are exothermic. the heat of reaction for the combustion of 2-methylheptane, c8h18, is 1.306×103 kcal/mol. what is the heat of combustion for 2-methylheptane in kcal/gram? 11.45 kcal/gram how much heat will be given off if molar quantities of 2-methylheptane react according to the following equation? 2 c8h18 + 25 o216 co2 + 18 h2o

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 20.06.2019 18:04, tashanicole
If this equation was completed which statement would it best support
Answers: 1

Chemistry, 22.06.2019 03:00, Dkhaurithompson
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Do you know the correct answer?
Combustion reactions are exothermic. the heat of reaction for the combustion of 2-methylheptane, c8h...
Questions in other subjects:




Chemistry, 12.03.2020 04:31



Social Studies, 12.03.2020 04:31

English, 12.03.2020 04:31