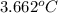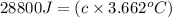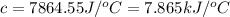Chemistry, 23.10.2019 02:00, michaellangley
The enthalpy of combustion of benzoic acid (c6h5cooh) which is often used to calibrate calorimeters, is −3227 kj/mol. when 1.09 g of benzoic acid was burned in a calorimeter, the temperature increased by 3.662◦c. what is the overall heat capacity of the calorimeter? the overall heat capacity includes the calorimeter hardware and the water that is in it. answer in units of kj/ ◦c.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, timiaparker
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 00:30, megaaan214p61pb7
Which compounds have the empirical formula ch2o? a. c2h4o2 b. c3h6o3 c. ch2o2 d. c5h10o5 e. c6h12o6
Answers: 3

Do you know the correct answer?
The enthalpy of combustion of benzoic acid (c6h5cooh) which is often used to calibrate calorimeters,...
Questions in other subjects:

World Languages, 11.01.2021 06:10

English, 11.01.2021 06:10


Mathematics, 11.01.2021 06:10




Mathematics, 11.01.2021 06:10

History, 11.01.2021 06:10

Mathematics, 11.01.2021 06:10


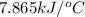
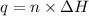
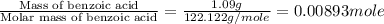
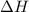 = enthalpy of combustion = 3227 kJ/mole
= enthalpy of combustion = 3227 kJ/mole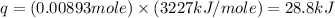
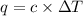
 = heat capacity of calorimeter = ?
= heat capacity of calorimeter = ? = change in temperature =
= change in temperature =