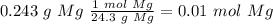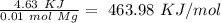When 0.243 g of mg metal is combined with enough hcl to make 100 ml of solution in a constant-pressure calorimeter, the following reaction occurs: mg1s2+2 hcl1aq2¡mgcl21aq2+h21g2if the temperature of the solution increases from 23.0 to 34.1 °c as a result of this reaction, calculate ∆h in kj> mol mg. assume that the solution has a specific heat of 4.18 j> g@°c and

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, tdowling331
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 03:30, isabelvaldez123
The boiling point of liquids is very high what does it indicate
Answers: 1


Chemistry, 22.06.2019 16:30, ddmoorehouseov75lc
Correct relationship between molecular formula and empirical formula
Answers: 1
Do you know the correct answer?
When 0.243 g of mg metal is combined with enough hcl to make 100 ml of solution in a constant-pressu...
Questions in other subjects:


English, 20.04.2020 23:55

History, 20.04.2020 23:55

World Languages, 20.04.2020 23:55




Mathematics, 20.04.2020 23:55


English, 20.04.2020 23:55


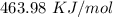
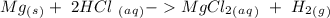
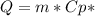 ΔT
ΔT
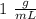
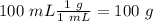
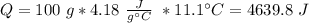
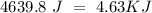
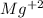 , so:
, so: