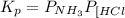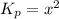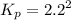Asample of solid ammonium chloride was placed in an evacuated chamber, and then heated causing it to decompose according to the following reaction: nh4cl(s) nh3(g) + hcl(g) in a particular experiment the pressure of nh3(g) in the container was found to be 2.2 atm. calculate the value of kp for the decomposition of nh4cl(s) at this temperature. kp = submitshow hints

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, angelinararr5783
Which answer lists the fundamental forces in order from strongest to weakest
Answers: 1

Chemistry, 22.06.2019 05:00, Angelanova69134
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 12:30, AnastasiaJauregui
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1
Do you know the correct answer?
Asample of solid ammonium chloride was placed in an evacuated chamber, and then heated causing it to...
Questions in other subjects:

English, 05.07.2019 13:30

History, 05.07.2019 13:30




Mathematics, 05.07.2019 13:30

Mathematics, 05.07.2019 13:30