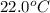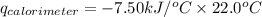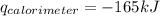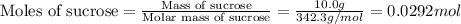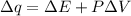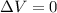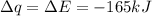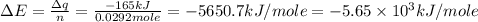Consider the reaction c12h22o11(s)+12o2(g)→12co2(g)+11h2o (l) in which 10.0 g of sucrose, c12h22o11, was burned in a bomb calorimeter with a heat capacity of 7.50 kj/∘c. the temperature increase inside the calorimeter was found to be 22.0 ∘c. calculate the change in internal energy, δe, for this reaction per mole of sucrose. express the change in internal energy in kilojoules per mole to three significant figures.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:20, Richwave17
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 23.06.2019 21:30, nsbegay2007
The conversion factor relating feet to meters is 1ft=0.305m. keep in mind that when using conversion factors, you want to make sure that like units cancel leaving you with the units you need. you have been told that a certain house is 164 m2 in area. how much is this in square feet?
Answers: 1

Chemistry, 23.06.2019 23:10, walkereddie580
Design a synthesis of 1-cyano-2-methylbicyclo[2.2.1]-2,5- heptadiene from cyclopentene and any other compounds using a diels-alder cycloaddition reaction. part 1 out of 9 choose the best option for the dienophile precursor to the target molecule.
Answers: 3

Chemistry, 24.06.2019 00:00, Cutiepie55561
When you push downward in earth to make a pole vault, earth exerts an equal force on the pole
Answers: 1
Do you know the correct answer?
Consider the reaction c12h22o11(s)+12o2(g)→12co2(g)+11h2o (l) in which 10.0 g of sucrose, c12h22o11,...
Questions in other subjects:


Mathematics, 05.02.2020 11:44

History, 05.02.2020 11:44




Physics, 05.02.2020 11:44



Mathematics, 05.02.2020 11:44


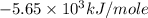
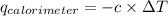
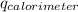 = heat released by calorimeter = ?
= heat released by calorimeter = ?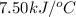
 = change in temperature of calorimeter =
= change in temperature of calorimeter =