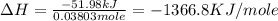Chemistry, 22.10.2019 23:30, hanacat6174
Find δh for the combustion of ethanol (c2h6o) to carbon dioxide and liquid water from the following data. the heat capacity of the bomb calorimeter is 34.65 kj/k and the combustion of 1.752 g of ethanol raises the temperature of the calorimeter from 294.42 k to 295.92 k .

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, lizzyhearts
What is the molality of a solution that has 4 mol of kci in 0.800 kg of water
Answers: 3

Chemistry, 21.06.2019 19:00, 21brooklynmartin
Does the temperature affect the solubility of sugar and salt in water? if it does tell me like different temperatures with different solubilities so i can sketch down a graph
Answers: 2

Chemistry, 22.06.2019 03:40, kellypechacekoyc1b3
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 10:00, sdlesley66
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1
Do you know the correct answer?
Find δh for the combustion of ethanol (c2h6o) to carbon dioxide and liquid water from the following...
Questions in other subjects:


Mathematics, 13.10.2020 02:01

Chemistry, 13.10.2020 02:01




Advanced Placement (AP), 13.10.2020 02:01


History, 13.10.2020 02:01

Mathematics, 13.10.2020 02:01


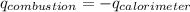
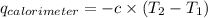
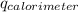 = heat released by calorimeter = ?
= heat released by calorimeter = ?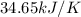
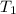 = initial temperature of calorimeter = 294.42 K
= initial temperature of calorimeter = 294.42 K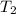 = final temperature of calorimeter = 295.92 K
= final temperature of calorimeter = 295.92 K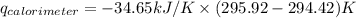
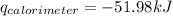
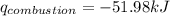
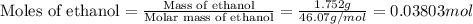
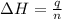
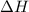 = enthalpy of combustion = ?
= enthalpy of combustion = ?