
A2.52-g sample of a compound containing only carbon, hydrogen, nitrogen, oxygen, and sulfur was burned in excess oxygen to yield 4.23 g of co2 and 1.01 g of h2o. another sample of the same compound, of mass 4.14 g, yielded 2.11 g of so3. a third sample, of mass 5.66 g, was burned under different conditions to yield 2.27 g of hno3 as the only nitrogen containing product. determine the empirical formula of the compound.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:00, raeprince9213
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 19:00, Farhan54019
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1

Chemistry, 23.06.2019 15:40, mackdoolittle1
Glucose (c6h12o6) is the simple sugar that plants make. what is the total number of atoms in glucose? 1 3 24 144
Answers: 1
Do you know the correct answer?
A2.52-g sample of a compound containing only carbon, hydrogen, nitrogen, oxygen, and sulfur was burn...
Questions in other subjects:

Biology, 14.11.2019 16:31


Mathematics, 14.11.2019 16:31


Mathematics, 14.11.2019 16:31



Mathematics, 14.11.2019 16:31


Social Studies, 14.11.2019 16:31


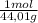 = 0,096 moles of CO₂ ≡ moles of C×12,01 g/mol = 1,15296 g of C/ 2,52*100 = 45,80 wt%
= 0,096 moles of CO₂ ≡ moles of C×12,01 g/mol = 1,15296 g of C/ 2,52*100 = 45,80 wt% 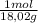 = 0,056 moles of H₂O = 0,112 moles of H×1,01 g/mol= 0,1132 g of H/2,52*100 = 4,49 wt%
= 0,056 moles of H₂O = 0,112 moles of H×1,01 g/mol= 0,1132 g of H/2,52*100 = 4,49 wt%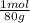 = 0,026 moles of SO₃ = 0,026 moles of S×32,065 g/mol= 0,8457 g of S/4,14*100 = 20,43 wt%
= 0,026 moles of SO₃ = 0,026 moles of S×32,065 g/mol= 0,8457 g of S/4,14*100 = 20,43 wt%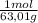 = 0,036 moles of H₂O = 0,036 moles of N×14 g/mol= 0,504g of H/5,56*100 = 9,07 wt%
= 0,036 moles of H₂O = 0,036 moles of N×14 g/mol= 0,504g of H/5,56*100 = 9,07 wt%



