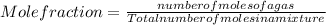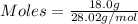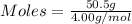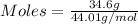Chemistry, 22.10.2019 02:00, jstringe424
Asample contains 18.0 g n2 (mw = 28.02 g/mol), 50.5 g he (mw = 4.00 g/mol), and 34.6 g co2 (mw = 44.01 g/mol). calculate the mole fraction of carbon dioxide in the sample.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 17:30, rollercoasterbuddies
Why is the melting of ice a physical change ?
Answers: 1

Chemistry, 22.06.2019 22:30, safiyabrowne7594
[ou.03jthe pictures below show the wavelengths and intensities of electromagnetic radiations emitted by three stars, star 1, star 2, and star 3. intensity intensity- intensity- 1000 3500 6000 8500 11000 wavelength (a) star 1 1000 3500 6000 8500 11000 1000 3500 6000 8500 11000 wavelength (a) wavelength (a) star 2 star 3 which of these statements is correct about the color of the three stars? star 2 is white in color o star 2 is yellow in color star 1 and star 3 are yellow in color star 1 and star 3 are white in color
Answers: 1
Do you know the correct answer?
Asample contains 18.0 g n2 (mw = 28.02 g/mol), 50.5 g he (mw = 4.00 g/mol), and 34.6 g co2 (mw = 44....
Questions in other subjects:

Mathematics, 03.02.2020 22:54

History, 03.02.2020 22:54

Physics, 03.02.2020 22:54


History, 03.02.2020 22:54

Computers and Technology, 03.02.2020 22:54

History, 03.02.2020 22:54


Chemistry, 03.02.2020 22:55

Physics, 03.02.2020 22:55