
Chemistry, 22.10.2019 01:00, maggiestevens5321
Calculate the amount of heat transferred when 24.0g of ch3oh(g) is decomposed by this reaction at constant pressure. for a given sample of ch3oh , the enthalpy change during the reaction is 82.6kj . what mass of methane gas is produced? how many kilojoules of heat are released when 38.7g of ch4(g) reacts completely with o2(g) to form ch3oh(g) at constant pressure?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:30, ansuaprajita1506
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 12:30, murtaghliam1
Word equation for k(s) +h2o(l) yield koh (aq) + h2
Answers: 3

Chemistry, 22.06.2019 14:30, belindajolete
How do temperature and salinity affect deepwater currents? as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2
Do you know the correct answer?
Calculate the amount of heat transferred when 24.0g of ch3oh(g) is decomposed by this reaction at co...
Questions in other subjects:

Mathematics, 19.03.2021 22:00

Mathematics, 19.03.2021 22:00

Computers and Technology, 19.03.2021 22:00

Mathematics, 19.03.2021 22:00


Mathematics, 19.03.2021 22:00

Mathematics, 19.03.2021 22:00




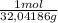 = 0,749 moles of CH₃OH(g)
= 0,749 moles of CH₃OH(g)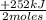 = 94,4 kJ
= 94,4 kJ = 0,656 moles of CH₄
= 0,656 moles of CH₄ = 10,5 g of CH₄
= 10,5 g of CH₄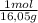 = 2,41 moles of CH₄(g)
= 2,41 moles of CH₄(g)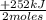 = 304 kJ
= 304 kJ



