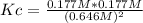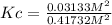Chemistry, 21.10.2019 18:10, lejeanjamespete1
Consider the reaction: 2hi(g) ⇄ h2(g) + i2(g). it is found that, when equilibrium is reached at a certain temperature, hi is 35.4 percent dissociated. calculate the equilibrium constant kc for the reaction at this temperature?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, melidacampos12
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2



Chemistry, 23.06.2019 06:30, madelineb6243
Which of these describes how heat is transferred by convection* a. sunlight travels through space without the aid of fluids or solids. b. warm air rises and takes the heat with it, eventually, it cools and sinks c. air at the equator rises and sinks at the poles. d. air molecules touch the warm ground, heating them up *not conduction
Answers: 3
Do you know the correct answer?
Consider the reaction: 2hi(g) ⇄ h2(g) + i2(g). it is found that, when equilibrium is reached at a c...
Questions in other subjects:


History, 28.06.2019 23:00

English, 28.06.2019 23:00

English, 28.06.2019 23:00

English, 28.06.2019 23:00

English, 28.06.2019 23:00

Mathematics, 28.06.2019 23:00

English, 28.06.2019 23:00


English, 28.06.2019 23:00


![Kc = \frac{[H2]*[I2]}{[HI]^2}](/tpl/images/0338/7477/a79bd.png)