Chemistry, 21.10.2019 13:10, roselyndiaz29
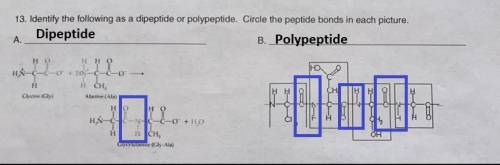
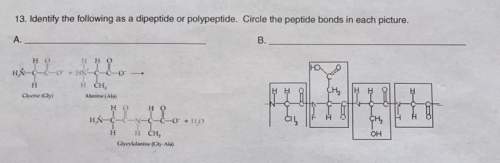
Identify the following as a dipeptide or polypeptide. circle the peptide bonds in each picture.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:20, evansh78
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

Chemistry, 23.06.2019 00:00, chloe8979
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1


Chemistry, 23.06.2019 10:10, Kennethabrown09
In a covalent bond, two atoms are held together by the attraction between . the number of covalent bonds that an atom can form depends on the number of in the atom.
Answers: 2
Do you know the correct answer?
Identify the following as a dipeptide or polypeptide. circle the peptide bonds in each picture.
Questions in other subjects:




English, 17.11.2020 01:00


Chemistry, 17.11.2020 01:00


Biology, 17.11.2020 01:00