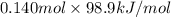Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, giusto1894
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 17:30, kevin72937
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 23.06.2019 02:00, hayleebeals50
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2

Chemistry, 23.06.2019 10:30, 7thaohstudent
What is the difference between skimming and absorbing methods of the oil removal
Answers: 2
Do you know the correct answer?
Calculate the amount of energy in the form of heat that is produced when a volume of 3.43 l of so2(g...
Questions in other subjects:


Mathematics, 04.03.2020 02:31

History, 04.03.2020 02:31

Mathematics, 04.03.2020 02:31

Mathematics, 04.03.2020 02:31


Social Studies, 04.03.2020 02:31

Mathematics, 04.03.2020 02:31



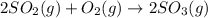
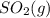 and
and 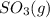 are as follows.
are as follows.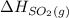 = -296.8 kJ/mol
= -296.8 kJ/mol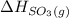 = -395.7 kJ/mol
= -395.7 kJ/mol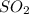 =
= 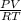
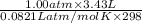 (as 1 bar = 1 atm (approximately))
(as 1 bar = 1 atm (approximately))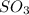 are also 0.140.
are also 0.140.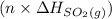 and
and 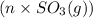
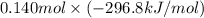 and
and 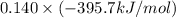 respectively.
respectively.![0.140 \times [-296.8 kJ/mol - (-395.7 kJ/mol)]](/tpl/images/0333/5672/320d5.png)