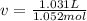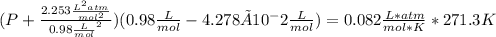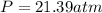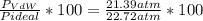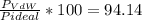1.according to the ideal gas law, a 1.052 mol sample of methane gas in a 1.031 l container at 271.3 k should exert a pressure of 22.72 atm. by what percent does the pressure calculated using the van der waals' equation differ from the ideal pressure? for ch4 gas,
a = 2.253 l2atm/mol2 and
b = 4.278×10-2 l/mol.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, grayfaith16
1) how many electrons are in each energy level of the following elements? a. he b. na c. na d. ne 2) how many valence electrons are percent in the following atoms? a. s b. mg c. be d. cl 3) which of the following elements are stable as atoms? a. he b. o c. cl d. ar if you are able to provide the work as to how you got the answers that would be greatly appreciated. : )
Answers: 1

Chemistry, 22.06.2019 17:00, brandiwingard
What is the mass of phosphorous in a 51-kg person
Answers: 1

Chemistry, 23.06.2019 06:10, ridzrana02
How can liquids be seperated by density a the liquids are absorbed onto a paper b the liquids are turned into seperate vapors c the liquids are collected as they evaporate d the liquids are allowed to seperate into layers
Answers: 1
Do you know the correct answer?
1.according to the ideal gas law, a 1.052 mol sample of methane gas in a 1.031 l container at 271.3...
Questions in other subjects:



Mathematics, 10.03.2022 14:00



Mathematics, 10.03.2022 14:00


English, 10.03.2022 14:00