
This question has multiple parts.
a. in a coffee-cup calorimeter, 1.81 g nh4no3 was mixed with 85.00 g water at an initial temperature 25.00oc. after dissolution of the salt, the final temperature of the calorimeter contents was 23.34oc.
assuming the solution has a heat capacity of 4.18 j/g∙oc, and assuming no heat loss to the calorimeter, calculate the enthalpy of solution (∆hsoln) for the dissolution of nh4no3 in units of kj/mol.
∆ = kj/mol
b. in a coffee-cup calorimeter, 1.81 g nh4no3 was mixed with 85.00 g water at an initial temperature 25.00oc. after dissolution of the salt, the final temperature of the calorimeter contents was 23.34oc.
if the enthalpy of hydration for nh4no3 is -630. kj/mol, calculate the lattice energy of nh4no3.
lattice energy = kj/mol

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, ruleolivas
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 22:30, eduardoguizar8787
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1


Chemistry, 23.06.2019 03:50, KAITLYN007
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer.
Answers: 1
Do you know the correct answer?
This question has multiple parts.
a. in a coffee-cup calorimeter, 1.81 g nh4no3 was mixed with...
a. in a coffee-cup calorimeter, 1.81 g nh4no3 was mixed with...
Questions in other subjects:


Mathematics, 21.01.2021 01:20


Chemistry, 21.01.2021 01:20

Mathematics, 21.01.2021 01:20



Mathematics, 21.01.2021 01:20


Mathematics, 21.01.2021 01:20


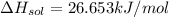

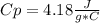
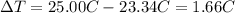
![m=m_{water} + m_{salt]=1.81g +85g= 86.81g](/tpl/images/0333/2995/cfc86.png)
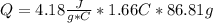
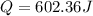
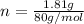
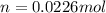
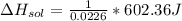
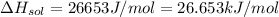
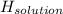 = 26.20 kJ/mol
= 26.20 kJ/mol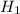 ) = - 656.20 kJ/mol
) = - 656.20 kJ/mol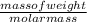
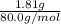

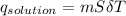
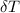 = 25.00°C - 23.34°C
= 25.00°C - 23.34°C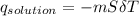 (since heat is lost by the water to the compound)
(since heat is lost by the water to the compound)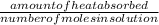
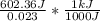
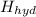 + Δ
+ Δ



