
Chemistry, 19.10.2019 00:30, hannahbannana98
You decide to titrate the solution of naoh using oxalic acid as a primary standard and phenolphthalein as the indicator. in the reaction of oxalic acid with naoh, every 1 mole of oxalic acid is deprotonated by 2 moles of naoh. the molecular weight of oxalic acid is 90.03 g/mol and you use 0.60 g in 50 ml water for the titration. you use 15.20 ml of your naoh solution in order to reach the endpoint of the titration. what is the concentration of your naoh solution?

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 18:50, christhegreat1
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 22.06.2019 19:30, youngdelvin123
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2
Do you know the correct answer?
You decide to titrate the solution of naoh using oxalic acid as a primary standard and phenolphthale...
Questions in other subjects:

Mathematics, 27.10.2019 16:43




Mathematics, 27.10.2019 16:43

Mathematics, 27.10.2019 16:43

Business, 27.10.2019 16:43


Mathematics, 27.10.2019 16:43

Mathematics, 27.10.2019 16:43


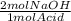 = 0.0133 mol NaOH
= 0.0133 mol NaOH



