
An herbicide contains only c, h, cl, and n. the complete combustion of a 200.0 mg sample of the herbicide in excess oxygen produced 209.2 ml of co2 and 122.0 ml of h2o vapor at stp. a separate analysis determined the 200.0 mg sample contained 55.14 mg cl. determine the percent composition of the herbicide.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, tdowling331
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 19:40, trodgers0202
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests. which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3


Chemistry, 23.06.2019 00:30, clairebear66
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1
Do you know the correct answer?
An herbicide contains only c, h, cl, and n. the complete combustion of a 200.0 mg sample of the herb...
Questions in other subjects:


Geography, 29.07.2021 08:00


English, 29.07.2021 08:00



Mathematics, 29.07.2021 08:00




 ×100 = 56,1%C
×100 = 56,1%C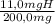 ×100 = 5,5%H
×100 = 5,5%H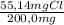 ×100 = 27,6%Cl
×100 = 27,6%Cl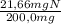 ×100 = 10,8%N
×100 = 10,8%N



