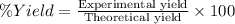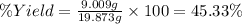Chemistry, 18.10.2019 23:30, blaze9889t
Alum is a compound used in a variety of applications including cosmetics, water purification, and as a food additive. it can be synthesized from aluminum metal, sulfuric acid, water, and potassium hydroxide, as seen in the equation. 2 al(s) 2 koh(aq) 4 h2so4(aq) 10 h2o(l) ⟶ 2 kal(so4)2∙12 h2o(s) 3 h2(g) alum using the data, determine the theoretical and percent yield for this alum synthesis. note that aluminum is the limiting reactant. description mass (g) bottle mass 10.221 bottle mass with aluminum pieces 11.353 final product and bottle mass 19.230 what is the theoretical yield of alum

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:00, applereams
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2

Chemistry, 22.06.2019 12:00, shifaxoxoxo
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

Chemistry, 22.06.2019 18:50, christhegreat1
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
Do you know the correct answer?
Alum is a compound used in a variety of applications including cosmetics, water purification, and as...
Questions in other subjects:

Mathematics, 02.09.2020 19:01


Physics, 02.09.2020 19:01



World Languages, 02.09.2020 19:01




History, 02.09.2020 19:01


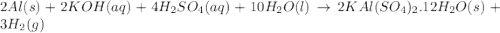
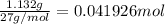
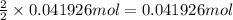 of alum.
of alum.