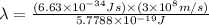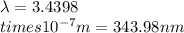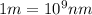Ultraviolet radiation and radiation of shorter wavelengths can damage biological molecules because they carry enough energy to break bonds within the molecules. a typical carbon–carbon bond requires 348 kj/mol to break. what is the longest wavelength of radiation with enough energy to break carbon–carbon bonds?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, lizethdominguez037
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 05:00, YoEsMyles3115
0.2348 grams of pbcl2 used to form 44.0 ml of solution.
Answers: 1

Chemistry, 22.06.2019 08:00, PrincessKeliah5538
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1
Do you know the correct answer?
Ultraviolet radiation and radiation of shorter wavelengths can damage biological molecules because t...
Questions in other subjects:

Mathematics, 09.01.2021 01:00

Mathematics, 09.01.2021 01:00


Mathematics, 09.01.2021 01:00

Social Studies, 09.01.2021 01:00






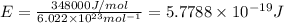
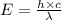
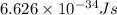
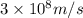
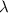 = wavelength of the radiation
= wavelength of the radiation