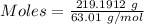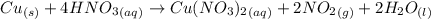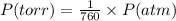Chemistry, 18.10.2019 19:10, densliverdensentos
Nitrogen dioxide is used industrially to produce nitric acid, but it contributes to acid rain and photochemical smog. what volume of nitrogen dioxide is formed at 724 torr and 28.2° c by reacting 4.84 cm3 of copper (d = 8.95 g/cm3) with 227 ml of nitric acid (d = 1.42 g/cm3, 68.0% hno3 by mass)?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, kkruvc
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1


Chemistry, 22.06.2019 15:00, Zagorodniypolina5
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2
Do you know the correct answer?
Nitrogen dioxide is used industrially to produce nitric acid, but it contributes to acid rain and ph...
Questions in other subjects:



Advanced Placement (AP), 19.10.2021 14:00

Mathematics, 19.10.2021 14:00


English, 19.10.2021 14:00



Chemistry, 19.10.2021 14:00

Chemistry, 19.10.2021 14:00


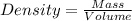
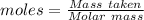
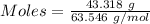
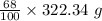 = 219.1912 g
= 219.1912 g