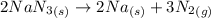Air bags are activated when a severe impact causes a steel ball to compress a spring and electrically ignite a detonator cap. this causes sodium azide (nan3) to decompose explosively according to the following reaction: 2nan3 (s) → 2na(s) + 3n2(g) what mass of nan3 must be reacted to inflate an air bag to 70.0 l at stp?

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 03:30, ilizzy1224
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 07:30, bbyniah123
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3
Do you know the correct answer?
Air bags are activated when a severe impact causes a steel ball to compress a spring and electricall...
Questions in other subjects:

History, 26.08.2021 20:40

Chemistry, 26.08.2021 20:40






History, 26.08.2021 20:40


Mathematics, 26.08.2021 20:40


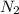 = 3.1214 moles
= 3.1214 moles