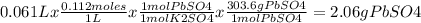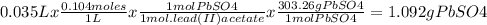Chemistry, 18.10.2019 19:00, chassidytjtrimb
A61.0ml sample of a 0.112m potassium sulfate solution is mixed with 35.0ml of a 0.104m lead(ii) acetate solution and the following precipitation reaction occurs:
k2so4(aq)+pb(c2h3o2)2(aq)? 2kc2h3o2(aq)+pbso4(s)
the solid pbso4 is collected, dried, and found to have a mass of 0.997g .
determine the limiting reactant, the theoretical yield, and the percent yield.
part a.
identify the limiting reactant.
kc2h3o2
pb(c2h3o2)2
k2so4
pbso4

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:30, MansellS5529
A3.37-mg sample of protein was chemically digested to convert its nitrogen into ammonia and then diluted to 100.0 ml. then 10.0 ml of this solution was placed in a 50-ml volumetric flask and treated with 5 ml of phenol solution plus 2 ml of sodium hypochlorite solution. the sample was diluted to 50.0 ml, and the absorbance at 625 nm was measured in a 1.00-cm cuvette and found to be 0.486. for reference, a standard solution was prepared from 10.0 mg of nh4cl (molar mass = 53.49 grams/mole) dissolved in 1.00 l of water. then 10.0 ml of this standard was placed in a 50-ml volumetric flask, treated in the same manner as the unknown, and the absorbance found to be 0.323. finally, a reagent blank was prepared using distilled water in place of unknown, it was treated in the same manner as the unknown, and the absorbance found to be 0.076. calculate the weight percent of nitrogen in the protein.
Answers: 1


Chemistry, 22.06.2019 22:10, steven0448
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1
Do you know the correct answer?
A61.0ml sample of a 0.112m potassium sulfate solution is mixed with 35.0ml of a 0.104m lead(ii) acet...
Questions in other subjects:

Health, 17.07.2019 20:20

Biology, 17.07.2019 20:20

Mathematics, 17.07.2019 20:20

Mathematics, 17.07.2019 20:20


Mathematics, 17.07.2019 20:20

History, 17.07.2019 20:20

Chemistry, 17.07.2019 20:20

English, 17.07.2019 20:20