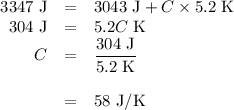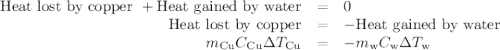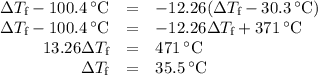Chemistry, 18.10.2019 17:10, tonimgreen17p6vqjq
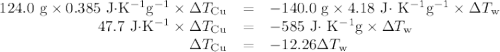
Acoffee-cup calorimeter contains 140.0 g of water at 25.1°c . a 124.0-g block of copper metal is heated to 100.4°c by putting it in a beaker of boiling water. the specific heat of cu(s) is 0.385 j/g⋅k. the cu is added to the calorimeter, and after a time the contents of the cup reach a constant temperature of 30.3°c .
(a)- determine the amount of heat, in j, lost by the copper block. enter the absolute amount of heat lost by cu, without a minus sign.
(b)- determine the amount of heat gained by the water. the specific heat of water is 4.18 j/g⋅k.
(c)-the difference between your answers for (a) and (b) is due to heat loss through the styrofoam → cups and the heat necessary to raise the temperature of the inner wall of the apparatus. the heat
capacity of the calorimeter is the amount of heat necessary to raise the temperature of the apparatus (the cups and the stopper) by 1 k. calculate the heat capacity of the calorimeter in j/k.
expressyour answer using two significant figures.
(d)-what would be the final temperature of the system if all the heat lost by the copper block were absorbed by the water in the calorimeter?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:30, kevin72937
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 22.06.2019 19:00, andrecoral105
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 22.06.2019 22:30, angelagonzalesownus1
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1
Do you know the correct answer?
Acoffee-cup calorimeter contains 140.0 g of water at 25.1°c . a 124.0-g block of copper metal is hea...
Questions in other subjects:

Mathematics, 24.05.2021 17:30



Mathematics, 24.05.2021 17:30

Mathematics, 24.05.2021 17:30

Mathematics, 24.05.2021 17:30

Law, 24.05.2021 17:30

Mathematics, 24.05.2021 17:30