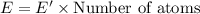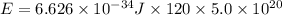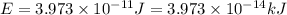Chemistry, 18.10.2019 03:30, Prettygirlyaya
What is the result in kilojoules of 5.0x10 atoms emitting energy for 2.00 minutes if plank's 1 x atom (hint: same hint as #13) ball part 3x10" 18 constant is 6.626 x 10-14

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, daytonalive83481
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 19:30, dorindaramirez0531
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1

Do you know the correct answer?
What is the result in kilojoules of 5.0x10 atoms emitting energy for 2.00 minutes if plank's 1 x ato...
Questions in other subjects:


Mathematics, 09.10.2019 06:30


Social Studies, 09.10.2019 06:30



Mathematics, 09.10.2019 06:30

History, 09.10.2019 06:30



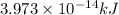 is the result in kiloJoules.
is the result in kiloJoules.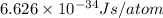

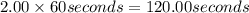
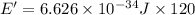
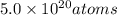 in 2.00 minutes be E.
in 2.00 minutes be E.