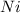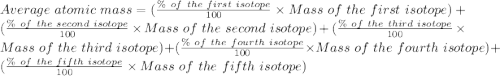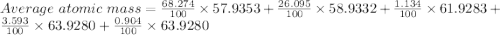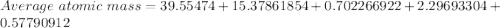Given the following information regarding the masses and relative abundances for the isotopes of an element, determine its atomic mass and its atomic symbol. (5 pts) isotope mass amu) relative abundance 57.9353 58.9332 60.9310 61.9283 63.9280 68.274% 26.095% 1.134% 3.593% 0.904%

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:20, kristieroth1
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1



Chemistry, 22.06.2019 14:30, villarrealc1987
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2
Do you know the correct answer?
Given the following information regarding the masses and relative abundances for the isotopes of an...
Questions in other subjects:


Mathematics, 31.10.2019 23:31


Mathematics, 31.10.2019 23:31


History, 31.10.2019 23:31

Social Studies, 31.10.2019 23:31



History, 31.10.2019 23:31