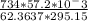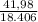At elevated temperatures, sodium chlorate decomposes to produce sodium chloride and oxygen gas. a 0.8765-g sample of impure sodium chlorate was heated until the production of oxygen gas ceased. the oxygen gas collected over water occupied 57.2 ml at a temperature of 22ºc and a pressure of 734 torr. calculate the mass percent of nahco₃ in the original sample. (at 22ºc the vapor pressure of water is 19.8 torr.)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:50, amandamac7339
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1


Chemistry, 23.06.2019 04:20, tatta95
Which activity describes an application of topographic maps? check all that apply. recreation, such as camping and hiking engineering, such as the construction of roads and buildings science, such as mapping stars in the sky business, such as analyzing population centers science, such as analyzing surface features
Answers: 1
Do you know the correct answer?
At elevated temperatures, sodium chlorate decomposes to produce sodium chloride and oxygen gas. a 0....
Questions in other subjects:

Computers and Technology, 26.02.2021 06:40

Biology, 26.02.2021 06:40


Chemistry, 26.02.2021 06:40



English, 26.02.2021 06:40



History, 26.02.2021 06:40