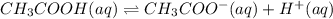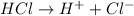Choose the statement below that is true.
a. a weak acid solution consists of mostly non-ioniz...

Chemistry, 17.10.2019 17:30, happy121906
Choose the statement below that is true.
a. a weak acid solution consists of mostly non-ionized acid molecules.
b. the term "strong electrolyte" means that the substance is extremely reactive.
c. a strong acid solution consists of only partially ionized acid molecules.
d. the term "weak electrolyte" means that the substance is inert.
e. a molecular compound that does not ionize in solution is considered a strong electrolyte.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, citlalli30
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 06:00, mapoohdoll
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 06:40, alyons60
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3
Do you know the correct answer?
Questions in other subjects:


Chemistry, 29.01.2020 08:46




History, 29.01.2020 08:46

Mathematics, 29.01.2020 08:46

English, 29.01.2020 08:46

Biology, 29.01.2020 08:46