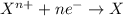As a result of the transfer of an electron from a less electronegative atom to a more electronegative atom,
a. the more electronegative atom is oxidized, and energy is released.
b. the more electronegative atom is reduced, and energy is consumed.
c. the more electronegative atom is oxidized, and energy is consumed.
d. the more electronegative atom is reduced, and energy is released.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:40, hardwick744
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

Chemistry, 22.06.2019 16:30, Eddie997
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 20:00, AaronEarlMerringer
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
Do you know the correct answer?
As a result of the transfer of an electron from a less electronegative atom to a more electronegativ...
Questions in other subjects:




Mathematics, 05.03.2021 21:30



Chemistry, 05.03.2021 21:30