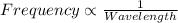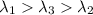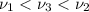One type of electromagnetic radiation has a frequency of 107.1 mhz, another type has a wavelength of 2.12 10-10 m, and another type of electromagnetic radiation has photons with energy equal to 3.97 10-19 j/photon. identify each type of electromagnetic radiation. 107.1 mhz 2.12 10-10 m 3.97 10-19 j/photon fm radiowaves x-rays visible (green) light visible (red) light fm radiowaves x-rays visible (green) light visible (red) light fm radiowaves x-rays visible (green) light visible (red) light rank them in order of increasing photon energy and increasing frequency

Answers: 3
Other questions on the subject: Chemistry




Do you know the correct answer?
One type of electromagnetic radiation has a frequency of 107.1 mhz, another type has a wavelength of...
Questions in other subjects:


Mathematics, 16.10.2020 17:01

Mathematics, 16.10.2020 17:01

Mathematics, 16.10.2020 17:01



Mathematics, 16.10.2020 17:01


Biology, 16.10.2020 17:01


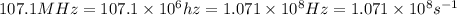
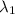
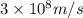
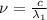
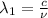
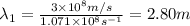
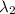
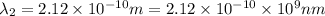
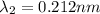 (X-rays)
(X-rays)
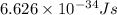
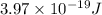
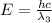
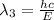
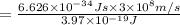
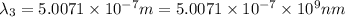
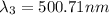 (visible light)
(visible light)