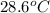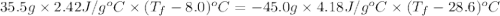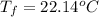Chemistry, 17.10.2019 06:00, steven2996
If 45.0 ml of ethanol (density = 0.789 g/ml) initially at 8.0 ∘c is mixed with 45.0 ml of water (density = 1.0 g/ml) initially at 28.6 ∘c in an insulated beaker, what is the final temperature of the mixture, assuming that no heat is lost? (cetoh=2.42j/(g⋅∘

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, alexmarche4675
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2


Chemistry, 22.06.2019 16:00, winnie45
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 17:40, adantrujillo1234
Areaction in which products can react to re-form reactants is
Answers: 1
Do you know the correct answer?
If 45.0 ml of ethanol (density = 0.789 g/ml) initially at 8.0 ∘c is mixed with 45.0 ml of water (den...
Questions in other subjects:


Chemistry, 31.01.2020 05:02


Social Studies, 31.01.2020 05:02

Social Studies, 31.01.2020 05:03

Health, 31.01.2020 05:03



Mathematics, 31.01.2020 05:03

Mathematics, 31.01.2020 05:03


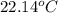
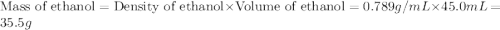
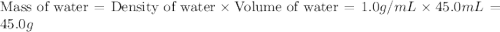
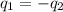
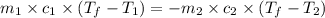
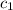 = specific heat of ethanol =
= specific heat of ethanol = 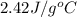
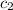 = specific heat of water =
= specific heat of water = 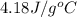
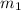 = mass of ethanol = 35.5 g
= mass of ethanol = 35.5 g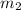 = mass of water = 45.0 g
= mass of water = 45.0 g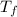 = final temperature of mixture = ?
= final temperature of mixture = ?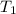 = initial temperature of ethanol =
= initial temperature of ethanol = 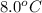
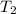 = initial temperature of water =
= initial temperature of water =