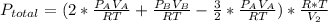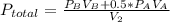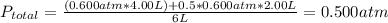Chemistry, 17.10.2019 05:20, janeekajones08
2. a 2.00 l flask containing 0.600 atm of gas a is connected to a 4.00 l flask containing 0.600 atm of gas b, after which the valve between the flasks is opened so that the gases can mix and react according to the following reaction equation. if the theoretical yield is produced at constant temperature, the total pressure in the combined flasks should 2a(g) + 3b(g) → a2b3(g)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, salvadorperez26
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2


Chemistry, 23.06.2019 17:10, jadeafrias
Which substance produces hydroxide ions in solution? a. an arrhenius acid b. an arrhenius base c. a brønsted-lowry acid d. a brønsted-lowry base e. an amphoteric substance
Answers: 3

Chemistry, 23.06.2019 19:30, micahsocool23
The molar mass of cu is 63.55 g/mol. the number of grams of cu produced in this reaction is .
Answers: 1
Do you know the correct answer?
2. a 2.00 l flask containing 0.600 atm of gas a is connected to a 4.00 l flask containing 0.600 atm...
Questions in other subjects:


SAT, 22.12.2021 04:20


SAT, 22.12.2021 04:20

Social Studies, 22.12.2021 04:20

Mathematics, 22.12.2021 04:20





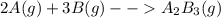
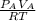
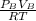
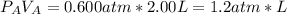

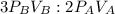
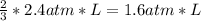 of A
of A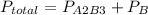
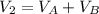
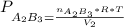
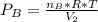
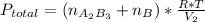
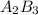
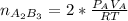
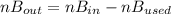
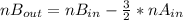
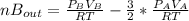
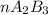 and nB in the total pressure equation we get:
and nB in the total pressure equation we get: