
Chemistry, 16.10.2019 22:00, nessuhbae6731
Suppose you have three monodisperse polymer samples, a, b, and c, with molar masses of 100,000 g/mol, 200,000 g/mol, and 300,000 g/mol, respectively. calculate the number average and weight-average molar mass of a blend obtained by mixing a, b, and c as follows: a) in equal proportions by weight b) in the molar ratio 1: 1: 1

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, willcohen42
150.0 grams of asf3 were reacted with 180.0 g of ccl4 to produce ascl3 and ccl2f2. if the actual yield of ccl2f2 was 127 g, what is the percent yield?
Answers: 2



Chemistry, 22.06.2019 19:00, hmontalvo22
How many moles are contained in 5.6 l of h2 at stp
Answers: 3
Do you know the correct answer?
Suppose you have three monodisperse polymer samples, a, b, and c, with molar masses of 100,000 g/mol...
Questions in other subjects:

Mathematics, 09.12.2020 17:50


Mathematics, 09.12.2020 17:50





History, 09.12.2020 17:50



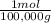 = 1x10⁻⁵ moles A
= 1x10⁻⁵ moles A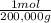 = 5x10⁻⁶ moles B
= 5x10⁻⁶ moles B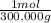 = 3,33x10⁻⁶ moles C
= 3,33x10⁻⁶ moles C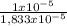 ×100 = 54,5%
×100 = 54,5%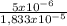 ×100 = 27,3%
×100 = 27,3%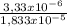 ×100 = 18,2%
×100 = 18,2%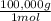 = 100,000g A
= 100,000g A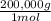 = 200,000g B
= 200,000g B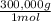 = 300,000g C
= 300,000g C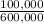 ×100 = 16,7%
×100 = 16,7%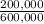 ×100 = 33,3%
×100 = 33,3%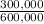 ×100 = 50,0%
×100 = 50,0%




