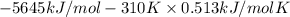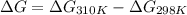Chemistry, 16.10.2019 04:00, Fatimaneedhelp
At 298 k the standard enthalpy of combustion of sucrose is -5645 kj/mol and the standard reaction gibbs energy is -5798 kj/mol. assume ∆h does not change to estimate the additional non-expansion work that may be obtained by raising the temperature to blood temperature, 37o c. enter your answer in kj/mol to two significant figures and do not enter the units.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, ebigham5117
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 14:30, neidaq12345
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 22.06.2019 18:00, sandeebassett3
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 23.06.2019 01:30, Dmoney5104
The biomedical technique in which a part of the brain is destroyed with electric current is known as a. electroconvulsive therapy b. prefrontal lobotomy c. bilateral cingulotomy d. tardive dyskinesia
Answers: 2
Do you know the correct answer?
At 298 k the standard enthalpy of combustion of sucrose is -5645 kj/mol and the standard reaction gi...
Questions in other subjects:

Physics, 10.02.2020 21:53








Mathematics, 10.02.2020 21:54

Mathematics, 10.02.2020 21:54


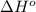 = -5645 kJ/mol
= -5645 kJ/mol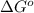 = -5798 kJ/mol
= -5798 kJ/mol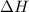 and
and 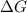 are as follows.
are as follows.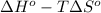
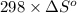
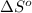 = 0.513 kJ/mol K
= 0.513 kJ/mol K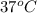 = (37 + 273) K = 310 K
= (37 + 273) K = 310 K