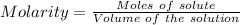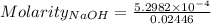Chemistry, 16.10.2019 01:30, jakails828
The concentration of a certain sodium hydroxide solution was determined by using the solution to titrate a sample of potassium hydrogen phthalate (abbreviated as khp). khp is an acid with one acidic hydrogen and a molar mass of 204.22 g/mol. in the titration, 24.46 ml of the sodium hydroxide solution was required to react with 0.1082 g khp. calculate the molarity of the sodium hydroxide.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:30, BornAdopted21
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2


Chemistry, 22.06.2019 12:10, coastieltp58aeg
Building glycogen from glucose molecules is an example of
Answers: 3
Do you know the correct answer?
The concentration of a certain sodium hydroxide solution was determined by using the solution to tit...
Questions in other subjects:


Health, 06.10.2019 01:30

Geography, 06.10.2019 01:30

Physics, 06.10.2019 01:30


Mathematics, 06.10.2019 01:30



Mathematics, 06.10.2019 01:30


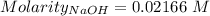
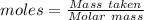
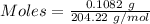
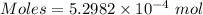
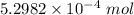 of KHP reacts with
of KHP reacts with