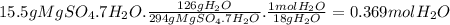Chemistry, 16.10.2019 01:30, Serenitybella
Bath salts such as epsom contain hydrate salts such as mgso 4 ·7h 2 o (magnesium sulfate heptahydrate). when heated, water is released as vapor and anhydrous mgso 4 remains. determine how many moles of vapor are released when 15.5 g of mgso 4 ·7h 2 o are heated. hint: include h 2 o when calculating the molar mass.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 04:20, milkshakegrande101
The equation below shows the reaction of zinc with hydrochloric acid (hcl). zn (s) + 2 hcl (aq) —> zncl2 (aq) + h2 (g) what will happen if the concentration of hcl is decreased? a. more zncl2 will be produced. b. the reaction rate will slow down. c. the hydrochloric acid will become more acidic. d. the reaction will produce water instead of hydrogen gas.
Answers: 1

Chemistry, 23.06.2019 05:20, cjking2320
Explain how global warming could have affected yellowstone frog and salamander habitat's, resulting in changes in the populations of these species
Answers: 2
Do you know the correct answer?
Bath salts such as epsom contain hydrate salts such as mgso 4 ·7h 2 o (magnesium sulfate heptahydrat...
Questions in other subjects:

Health, 31.03.2020 20:58



Mathematics, 31.03.2020 20:58




Physics, 31.03.2020 20:58

Mathematics, 31.03.2020 20:58