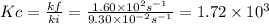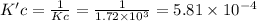At a given temperature, the elementary reaction a > b in the forward direction is first order in a with a rate constant of 1.60*10^2 s^-1. the reverse reaction is first order in b and the rate constant is 9.30*10^-2 s^-1what is the value of the equilibrium constant for the reaction a > b at this temperature? what is the value of equilibrium constant for the reaction b--> a at this temperature?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:50, bellojamilet410
What substance has a mass of 9.5g and volume of 2.1cm^3
Answers: 2

Chemistry, 23.06.2019 01:00, williedenmark42
What is the most common form of matter in the universe
Answers: 2

Do you know the correct answer?
At a given temperature, the elementary reaction a > b in the forward direction is first order in...
Questions in other subjects:

Mathematics, 26.02.2021 23:20

Mathematics, 26.02.2021 23:20



Mathematics, 26.02.2021 23:20

Mathematics, 26.02.2021 23:20


Mathematics, 26.02.2021 23:20

Chemistry, 26.02.2021 23:20