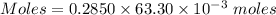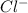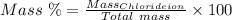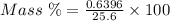Chemistry, 16.10.2019 00:00, Greghairston4839
The mass percent of cl⁻ in a seawater sample is determined by titrating 25.00 ml of seawater with agno₃ solution, causing a precipitation reaction. an indicator is used to detect the end point, which occurs when free ag⁺ ion is present in solution after all the cl⁻ has reacted. if 63.30 ml of 0.2850 m agno₃ is required to reach the end point, what is the mass percent of cl⁻ in the seawater (d of seawater = 1.024 g/ml)?

Answers: 2
Other questions on the subject: Chemistry




Chemistry, 23.06.2019 04:20, monifaWilson
The graph shows one consequence of urban sprawl. how did urban sprawl contribute to the change in biodiversity
Answers: 2
Do you know the correct answer?
The mass percent of cl⁻ in a seawater sample is determined by titrating 25.00 ml of seawater with ag...
Questions in other subjects:

History, 31.12.2021 14:00



Mathematics, 31.12.2021 14:00

Business, 31.12.2021 14:00

Biology, 31.12.2021 14:00

Mathematics, 31.12.2021 14:00




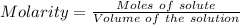
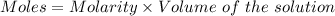
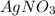 :
: