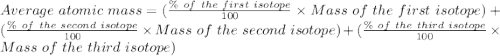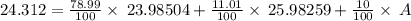Chemistry, 15.10.2019 21:20, francisco42002
Naturally occurring magnesium has an atomic mass of 24.312 and consists of three isotopes. the major isotope is mg24, natural abundance 78.99%, relative atomic mass 23.98504. the next most abundant isotope is mg26, relative atomic mass 25.98259. the third most abundant isotope is mg25 whose natural abundance is in the ratio of 0.9083 to that of mg26. find the relative atomic mass of mg25.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 01:00, kaykardash
Which polymers are most closely related? a. protein and nucleic acids b. cellulose and starch c. nucleic acids and starch d. nucleic acids and cellulose
Answers: 2

Chemistry, 23.06.2019 06:30, aurikmah2005
Acertain atom has 22 protons and 19 electrons. this atom loses an electron. the net charge on the atom is now 4+1+01-4-. if this same atom with 22 protons and 19 electrons were to gain 3 electrons, the net charge on the atom would be 3+2+02-3-.
Answers: 1
Do you know the correct answer?
Naturally occurring magnesium has an atomic mass of 24.312 and consists of three isotopes. the major...
Questions in other subjects:


Chemistry, 16.06.2021 06:10

Mathematics, 16.06.2021 06:10



Mathematics, 16.06.2021 06:10