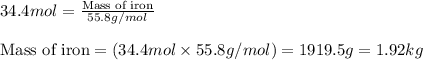Chemistry, 15.10.2019 21:10, jojo887314
Before arc welding was developed, a displacement reaction involving aluminum and iron(iii) oxide was commonly used to produce molten iron (the thermite process). this reaction was used, for example, to connect sections of iron railroad track. calculate the mass of molten iron produced when 2.28 kg of aluminum reacts with 17.2 mol of iron(iii) oxide.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, Killion2022
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1


Chemistry, 22.06.2019 16:30, Eddie997
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2
Do you know the correct answer?
Before arc welding was developed, a displacement reaction involving aluminum and iron(iii) oxide was...
Questions in other subjects:



Physics, 19.12.2020 02:30


Physics, 19.12.2020 02:30


Mathematics, 19.12.2020 02:30


Mathematics, 19.12.2020 02:30


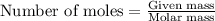 ......(1)
......(1)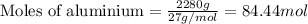
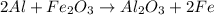
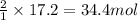 of aluminium
of aluminium