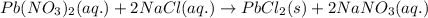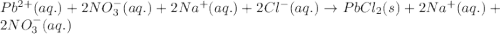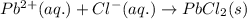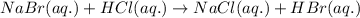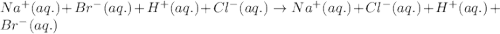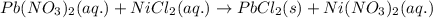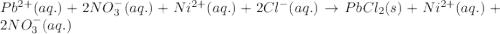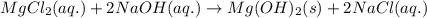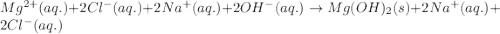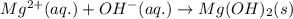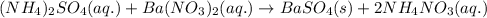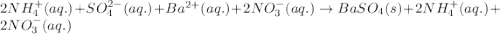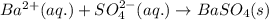Chemistry, 15.10.2019 19:30, davgre1271
Determine net ionic equations, if any, occuring when aqueous solutions of the following reactants are mixed. select "true" or "false" to indicate whether or not the stated reaction (or "no reaction") correctly corresponds to the expected observation in each case. lead(ii) nitrate and sodium chloride; no reaction occurs. sodium bromide and hydrochloric acid; no reaction occurs. nickel(ii) chloride and lead(ii) nitrate; pb2+(aq) + 2cl-(aq) --> pbcl2(s) magnesium chloride and sodium hydroxide; no reaction occurs. ammonium sulfate and barium nitrate; ba2+(aq) + so42-(aq) --> baso4(s)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, speris1443
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 13:10, Jana1517
The last few miles of the marathon are the most difficult for heather, her hair plastered to her head, sweat clinging to her arms, and her legs already feeling as if they had nothing left, just dead weight. after grabbing a cup of ice water, she feels the ice cubes smash against her nose as she gulps some cool refreshment and keeps on running. in these last few miles, the breeze kicks up and she finally feels some coolness against her skin. drips of sweat, once clinging to her forehead, now spill down, and heather feels more pain as the sweat flows into her eyes. which of the following is the most likely reason why the ice struck heather’s nose when she took a drink? a) water can function as a solvent. b) water can store large amounts of heat. c) water can moderate temperatures through evaporative cooling. d) the density of water decreases when it freezes. e) water has a cohesive nature. sweat remained on heather’s forehead and arms because of the a) high salt content of sweat b) cohesive nature of water c) ability of water to moderate heat d) high evaporative cooling effect of water e) ability of water to act as a solvent
Answers: 1

Chemistry, 22.06.2019 14:00, jivsf
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3
Do you know the correct answer?
Determine net ionic equations, if any, occuring when aqueous solutions of the following reactants ar...
Questions in other subjects:









Mathematics, 09.03.2021 02:30

Mathematics, 09.03.2021 02:30