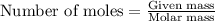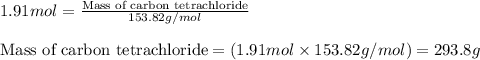Freon−12 (cf2cl2), widely used as a refrigerant and aerosol propellant, is a dangerous air pollutant. in the troposphere, it traps heat 25 times as effectively as co2, and in the stratosphere, it participates in the breakdown of ozone. freon−12 is prepared industrially by reaction of gaseous carbon tetrachloride with hydrogen fluoride. hydrogen chloride gas also forms. how many grams of carbon tetrachloride are required for the production of 28.5 dm3 of freon−12 at 21°c and 1.62 atm?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, fvmousdiana
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3


Chemistry, 22.06.2019 22:00, notearslefttocry14
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2

Do you know the correct answer?
Freon−12 (cf2cl2), widely used as a refrigerant and aerosol propellant, is a dangerous air pollutant...
Questions in other subjects:


Mathematics, 11.07.2019 06:00

Mathematics, 11.07.2019 06:00

History, 11.07.2019 06:00


Spanish, 11.07.2019 06:00



Mathematics, 11.07.2019 06:00


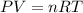
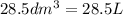 (Conversion factor:
(Conversion factor: 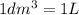 )
)![21^oC=[21+273]K=294K](/tpl/images/0322/3709/32d52.png)
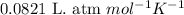
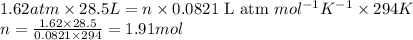
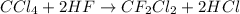
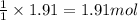 of carbon tetrachloride
of carbon tetrachloride