
Chemistry, 15.10.2019 04:30, smmailloux2335
Consider a reactant with an order of 1: what effect does that reactant have on the rate equation when the concentration is doubled 1) the reaction rate is double with respect to that reactant 2) the order doesnt effect the reaction so there will be no change 3) the reaction rate quadruples with respect to that reactant. 4) the reaction rate isn't changed by individual reactants so there will be no change.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, flowergirly34
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1


Chemistry, 22.06.2019 05:20, anggar20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1

Chemistry, 22.06.2019 11:00, familyvazquez7
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3
Do you know the correct answer?
Consider a reactant with an order of 1: what effect does that reactant have on the rate equation wh...
Questions in other subjects:

Mathematics, 04.05.2021 14:10

English, 04.05.2021 14:10

Mathematics, 04.05.2021 14:10

Mathematics, 04.05.2021 14:10

World Languages, 04.05.2021 14:10



Mathematics, 04.05.2021 14:10


Mathematics, 04.05.2021 14:10


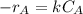
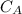 power is one (order 1), this could be proved just by checking it out in the rate law.
power is one (order 1), this could be proved just by checking it out in the rate law.



