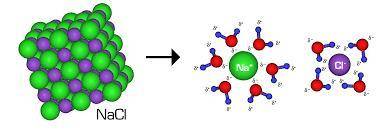
How does dissolving an ionic compound in water increase electrical conductivity?
a. the...

Chemistry, 15.10.2019 00:00, hilzepesqtatiana
How does dissolving an ionic compound in water increase electrical conductivity?
a. the water molecules electrostatically interact with the ions in the crystal and let the ions move freely.
b. the water molecules apply heat to break the ionic bonds in the crystal and let the ions move freely.
c. the water molecules chemically react with the ions in the crystal and let the ions move freely.
d. the water molecules remove heat to break the ionic bonds in the crystal and let the ions move freely.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:00, tcapele252
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 19:30, toriabrocks
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2


Chemistry, 22.06.2019 23:00, brianfranklin17
What is the correct lewis dot structure for arsenic?
Answers: 2
Do you know the correct answer?
Questions in other subjects:

Arts, 23.07.2020 17:01