Chemistry, 14.10.2019 21:30, Affousietta
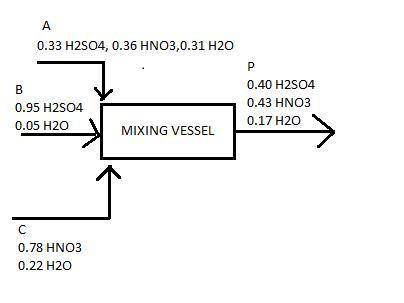
The spent acid from a nitration process contains 33% h2so4, 36% hno3 and 31% h2o. this is to be strengthened by the addition of concentrated h2so4 containing 95% h2so4 and nitric acid containing 78% hno3. the final strengths of mixed acid solution contains 40% h2so4 43% hno3 and the rest water. calculate : a. the amount of spent acid
b. the amount of concentrate h2so4
c. thr amount of concentrate hno3 to be mixed to produce 1500 kg of tge desired acid

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 21:00, advancedgamin8458
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2

Chemistry, 21.06.2019 21:00, sophiapknight
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 21.06.2019 23:30, lylessd423
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2
Do you know the correct answer?
The spent acid from a nitration process contains 33% h2so4, 36% hno3 and 31% h2o. this is to be stre...
Questions in other subjects:


English, 27.10.2020 01:00

Mathematics, 27.10.2020 01:00