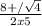Chemistry, 14.10.2019 21:00, missdee6929
H2(g) + i2(g) â 2 hi(g) kc = 64 at 400âºc 3.00 moles of h2 and 3.00 moles of i2 are placed in a 4.00 l container and the system is allowed to reach equilibrium. calculate the concentration of hi at equilibrium.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, jabper5522
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 04:00, breannaasmith1122
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1


Chemistry, 22.06.2019 20:30, ShahinF7536
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1
Do you know the correct answer?
H2(g) + i2(g) â 2 hi(g) kc = 64 at 400âºc 3.00 moles of h2 and 3.00 moles of i2 are placed in a 4.00...
Questions in other subjects:

Mathematics, 04.11.2020 20:10

Mathematics, 04.11.2020 20:10





Mathematics, 04.11.2020 20:10

History, 04.11.2020 20:10



![Kc = \frac{{D}^dx[C]^c}{[A]^ax[B]^b}](/tpl/images/0319/5603/47fdd.png)
![Kc = \frac{[HI]^2}{[H2]x[I2]}](/tpl/images/0319/5603/fe065.png)